Pre-clinical Trials
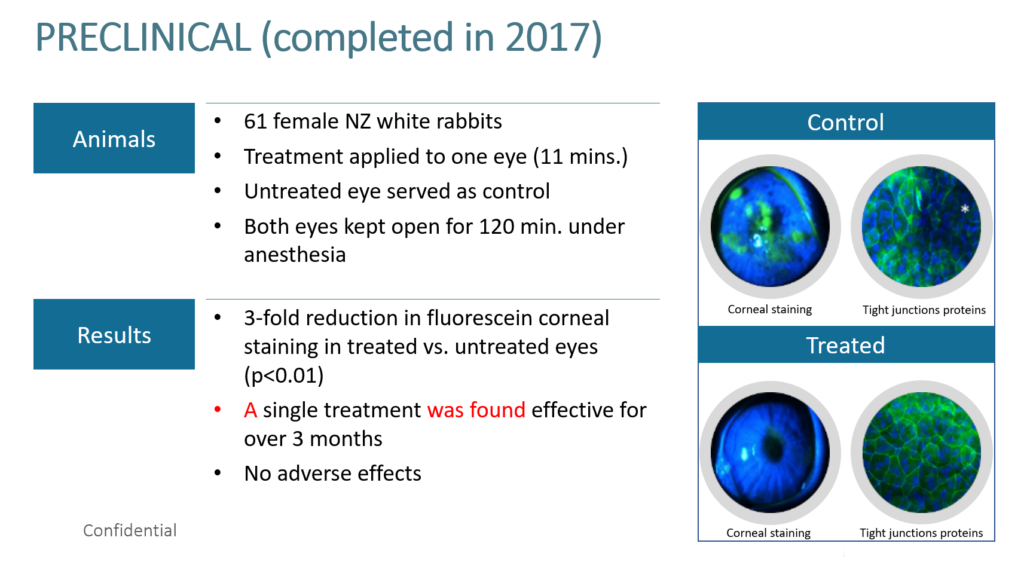
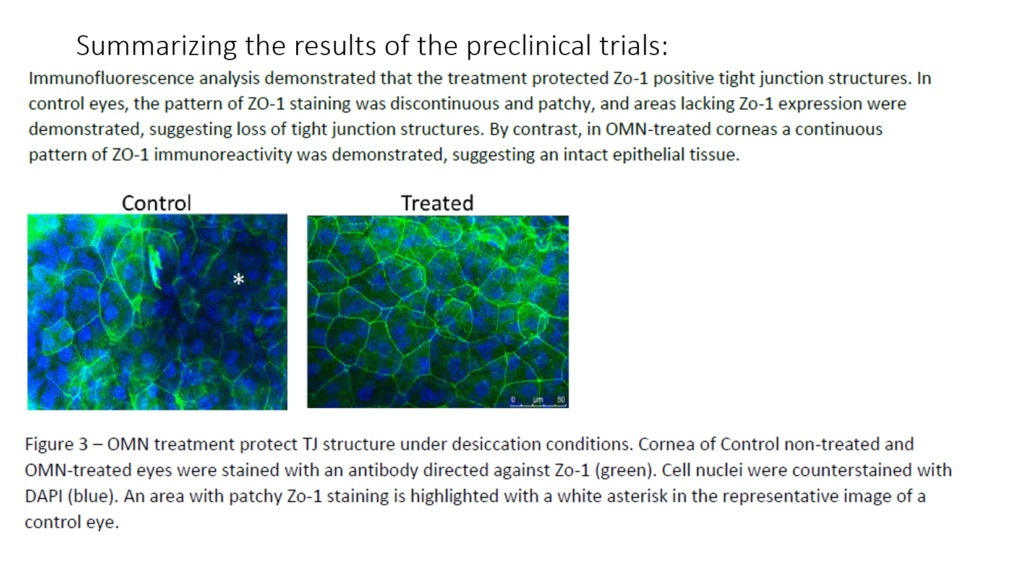
Epitech completed pre-clinical trials in 2017 and published the results in 2020 which demonstrated that RMS treatment decreases epithelial corneal erosions in a rabbit model of exposure keratopathy, with no indication of pathological changes.
First in Human (FIH) Clinical Trials
First In Human (FIH) Clinical Trial
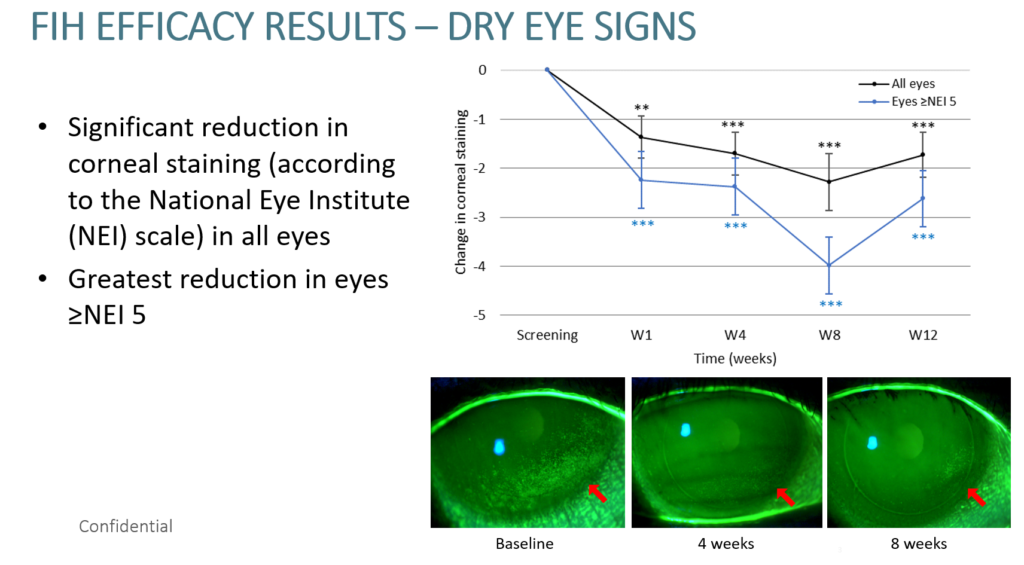
Two pre-pilot clinical studies have been completed. The first included 50 patients with moderate to severe dry eye disease and the second study (also with 50 patients) included a placebo or “sham” control group. Both studies were performed at three world renowned international sites without any adverse events or changes in safety-related endpoints or vital signs. The efficacy results showed significant reduction in corneal staining (a sign of dryness) in all eyes as well as an improved reported quality of life and an average reduction of 55% in lubricant use.
“A single session of Ocular Magnetic Neurostimulation treatment was safe, well-tolerated, and induced substantial reduction of corneal epithelium defects.”
Epitech is in the process of working with the FDA to determine the necessary requirements for the next study to be ready to submit data for 510(k) clearance.